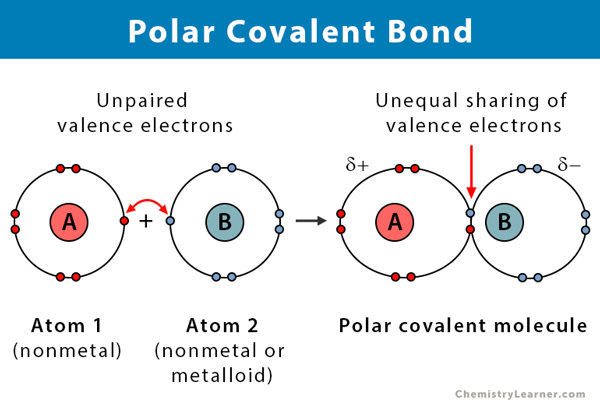
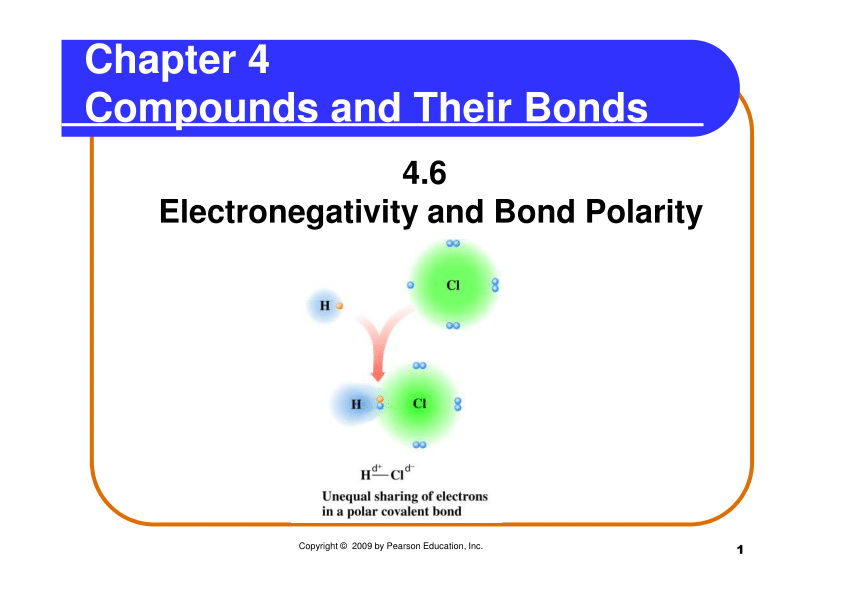
Describe a Polar Covalent Bond Using the Term Electronegativity
What does the term amino acid signify about the structure of the molecule. Hydroxonium ions H 3 O are produced.
Sulfate is the spelling recommended by IUPAC but sulphate was traditionally used in British English.
. Hydrogen bonds are much weaker than covalent bonds only about 5 to 10 as strong but are generally much stronger than other dipole-dipole attractions and dispersion forces. A polar covalent bond a nonpolar covalent bond. 10 Describe the impact of World War II on the lives of American citizens including wartime economic measures population shifts growth in the middle class growth of industrialization advancements in science and technology increased wealth in the African-American community racial and ethnic tensions Servicemens Readjustment Act of 1944.
In methane carbon has a valence of 4. When the difference between the electronegativities of the elements in a compound is relatively large the compound is best classified as ionic. This module provides a foundation for considering states of matter in all their complexity.
When it comes to different liquids some mix well while others dont. In water oxygen has a valence of 2. Step 1 Figuring out the total number of valence electrons in the molecule is the first and most remarkable step.
Although the structures of the functional groups important to life hydroxyl carbonyl carboxyl amino and phosphate vary in chemical structure they share one thing in common. The sulfur atom is in the 6 oxidation state while the four oxygen atoms are each in. Using both words and drawings at the microscopic level explain the characteristics of each of the following bonds.
A polar molecule is one that has a greater. Electrons are pulled harder by oxygen. Describe a polar molecule.
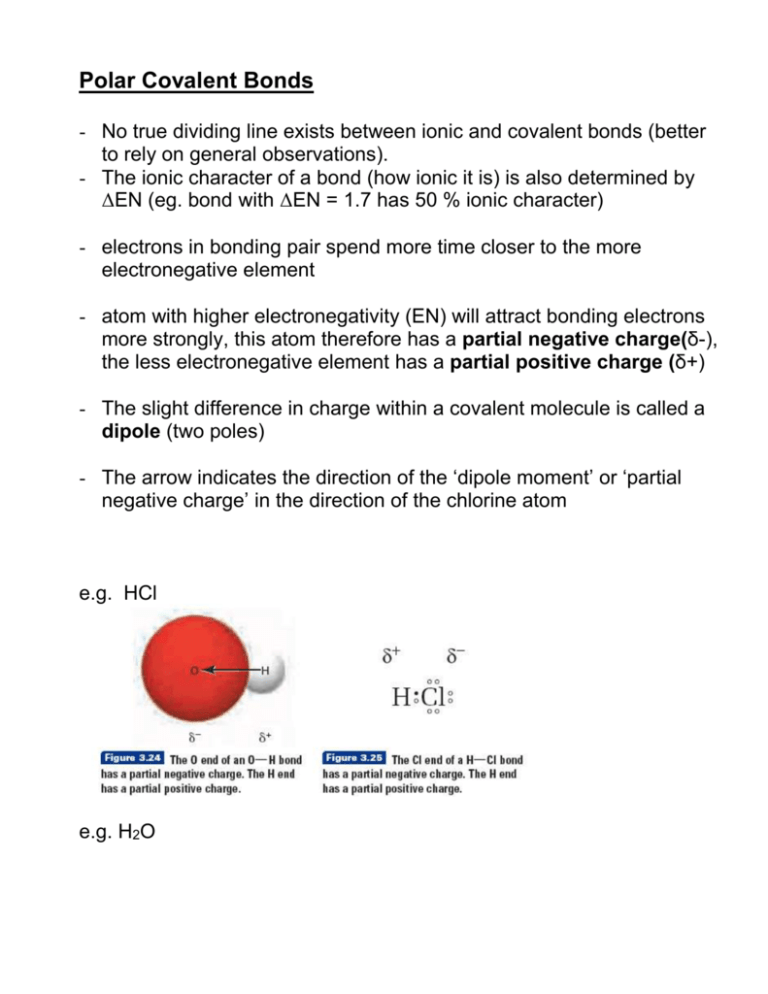
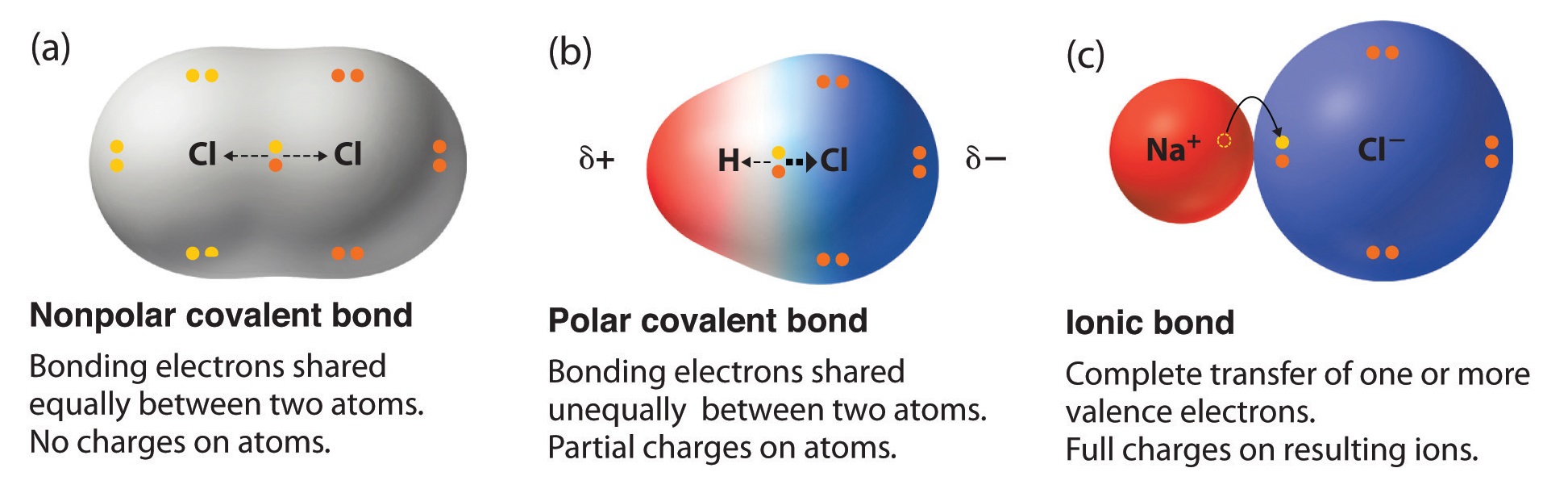
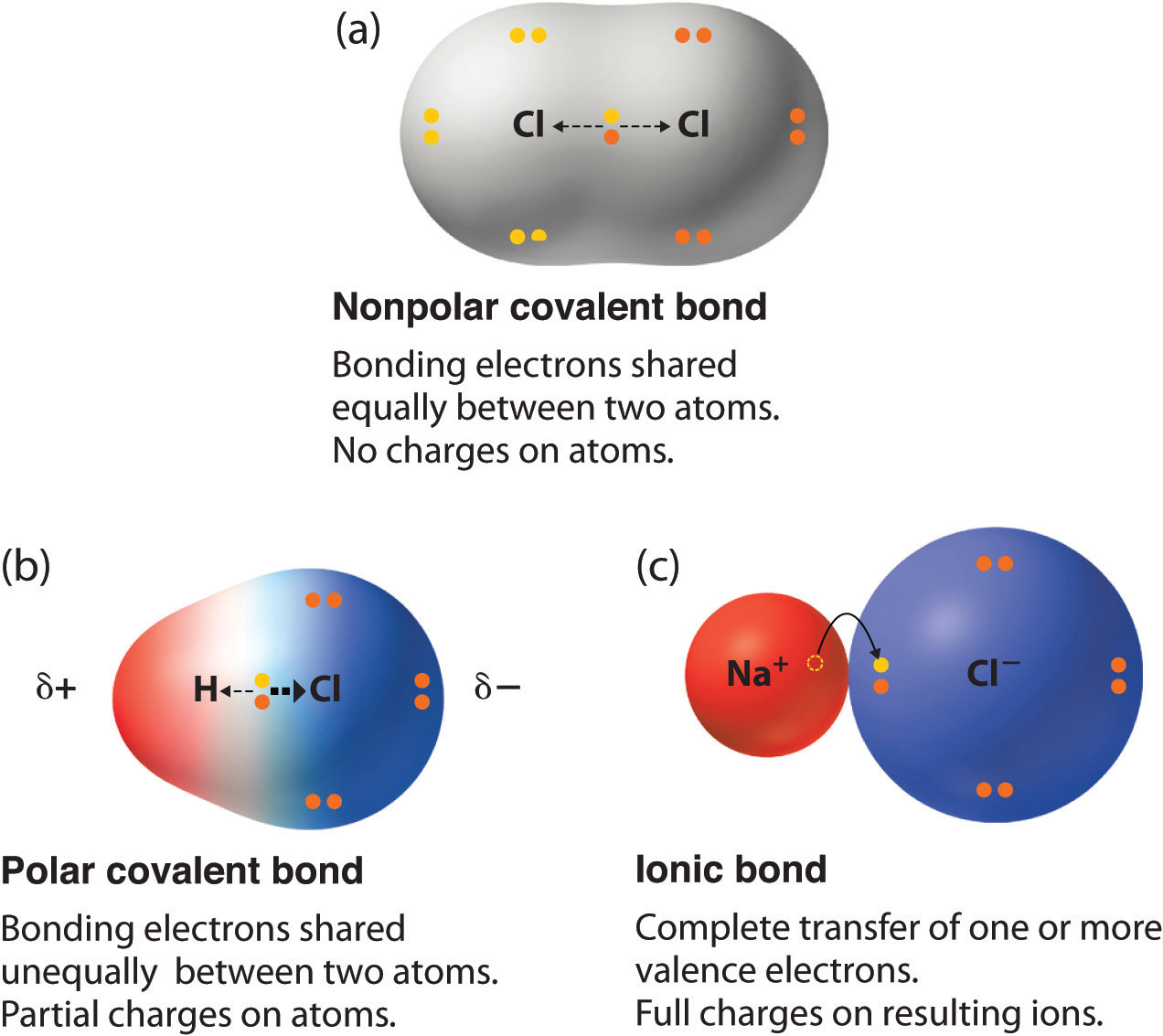
- the bond between oxygen and hydrogen in water H2O - the bond between nitrogen atoms in diatomic nitrogen N2 - the atoms in a covalent bond have partial charges - electrons are not equally shared by the atoms in a covalent bond. Using Electronegativity to Identify Ionic Covalent and Polar Covalent Compounds. NaCl LiF and SrBr 2 are good examples of ionic compounds.
The electrons are pulled toward one of the atoms generating partial charges on the ends. A co-ordinate dative covalent bond is formed between one of the lone pairs on the oxygen and the hydrogen from the HCl. Access the answers to hundreds of The periodic table questions that.
Start studying Chemistry Unit 5 - The Mole. 2 Describe the information a molecular formula provides. And in hydrogen chloride chlorine has a valence of 1Chlorine as it has a valence of one can be substituted for.
Explain with the help of suitable example polar covalent bond. Despite use of the word bond keep in mind that hydrogen bonds are intermolecular attractive forces not intramolecular attractive forces covalent bonds. In ammonia nitrogen has a valence of 3.
1 and Example 12. The atom with the highest number of binding locations is the central atom. A molecule is a group of atoms that associates.
It explains the basic properties of liquids and explores how intermolecular forces determine their behavior. A sign implies losing electrons and - means gaining. Electronegativity is the tendency of an atom to attract shared pair of electrons.
A polar covalent bond has unequal sharing of electrons. Two characteristics other than electronegativity also have an important influence on the chemical behavior of these compounds. The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement.
Electrons spend more time orbiting oxygen. Step 2 Next thing is determining the central atom. If you had 1000 carbon atoms and pulled out just one atom what would its Chemistry unit 1 packet answers AP Chemistry.
The combining capacity or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. Classify each statement or molecule as a polar bond or nonpolar bond. Some pour quickly while others flow slowly.
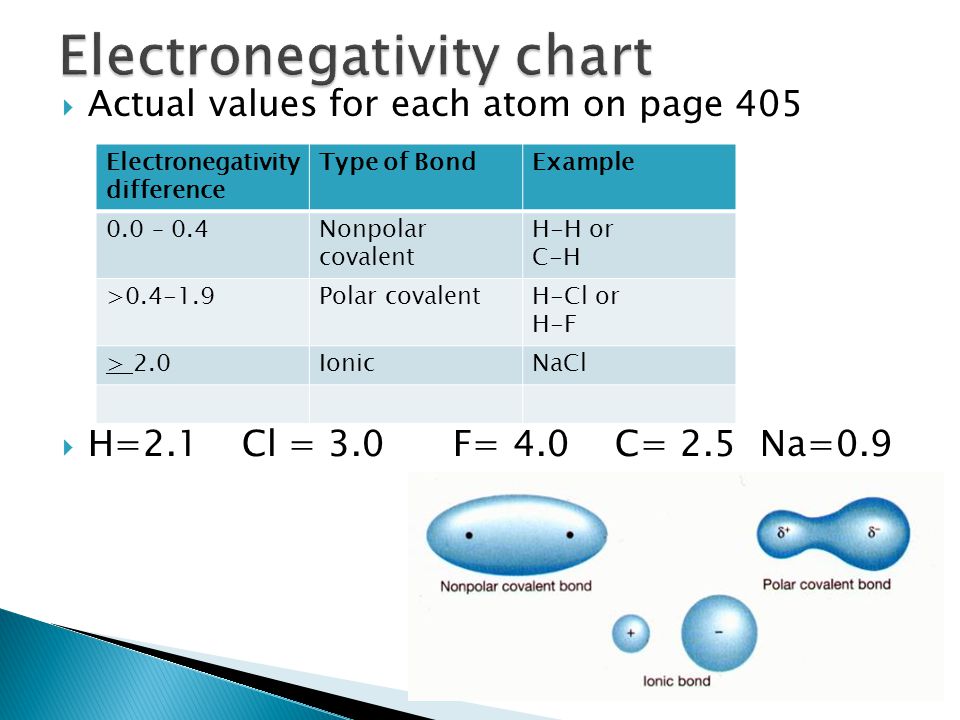
Get help with your The periodic table homework. The strongest of the carbon-halogen covalent bonds is that to fluorine. The greater the electronegativity difference the greater the ionic nature of the bond.
It is the property of an isolated atom. The concepts of cohesion adhesion and viscosity are defined. Remarkably this is the strongest common single bond to carbon being roughly 30 kcalmole stronger.
1 g of oxygen. If you arent sure about co-ordinate bonding you should follow this link. The bond is polar.
Choose all that apply a. The enthalpy of a given molecular interaction between two non-bonded atoms is 1 - 10 kcalmole 4 - 42 kjoulemole which in the lower limit is on the order of RT and in the upper limit is significantly less than a covalent bond. The Periodic Table Questions and Answers.
The first of these is covalent bond strength. While doing so do take care of the signs. It is the property of bonded atom.
In each case the electronegativity of the. The O-H bond in methyl amine is less polarized as shown by the lighter blue color around the hydrogen making it less acidic than methanol. Bonds hold atoms together within molecules.
Tell students to turn to pages 2425 in the Student Workbook and read the information. In a covalent bond between carbon and oxygen. The oxygen will have a net partial negative charge.
The symmetry is the same as that of methane. Describe a polar covalent bond. However the C-H bond in ethane lack virtually any polarity as shown by the lack of a blue color making it non-acidic.
Whereas electron gain enthalpy is the tendency of an atom to attract outside electron. It consists of an amino group and a carboxyl group.
What Is A Polar Covalent Bond Quora

Polar Covalent Bond Definition And Examples

Polar And Nonpolar Covalent Bonds Definitions Molecules And Examples

6 4 Polarity Of Molecules Introductory Chemistry
Polar Covalent Bonds And The Electronegativity

4 2 Polar And Non Polar Covalent Bonds Sl Youtube
8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts
Polar And Nonpolar Covalent Bonds Characteristics Differences

2 1 Polar Covalent Bonds Electronegativity Flashcards Quizlet
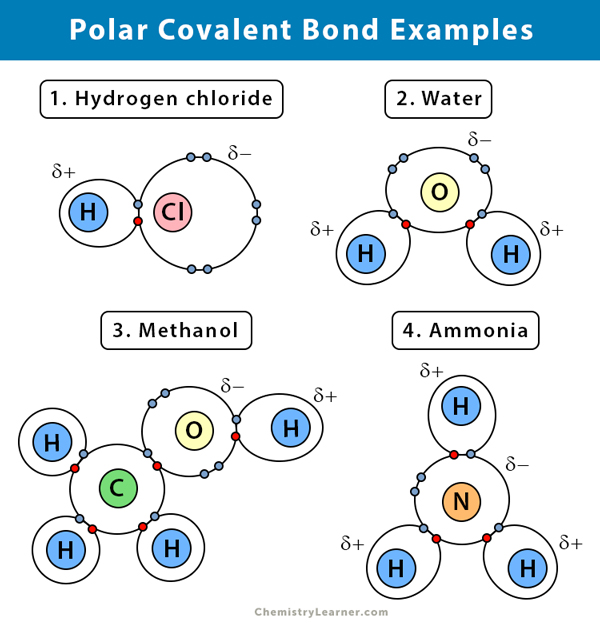
Polar Covalent Bond Definition And Examples
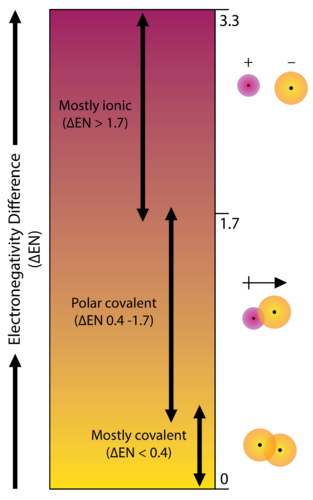
Bond Polarity Chemistry For Non Majors

Bond Polarity Chemistry For Non Majors

Polar Vs Nonpolar Covalent Bonds Examples What Are Polar Nonpolar Covalent Bonds Video Lesson Transcript Study Com

What Is A Polar Covalent Bond Quora
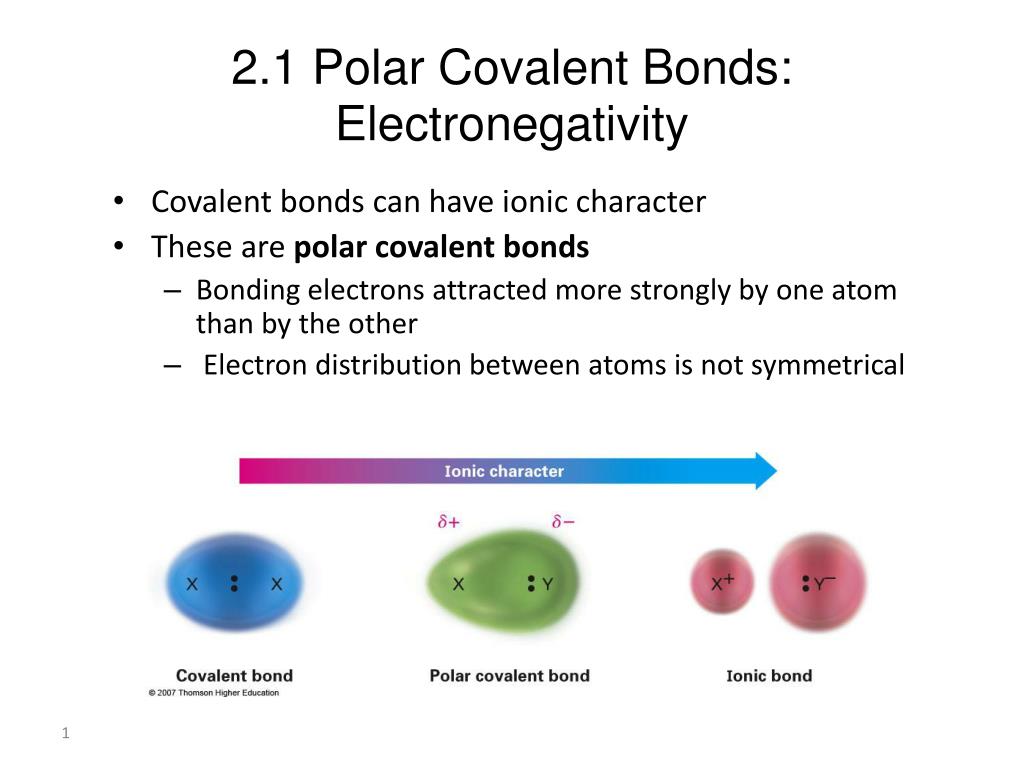
Ppt 2 1 Polar Covalent Bonds Electronegativity Powerpoint Presentation Id 2237583
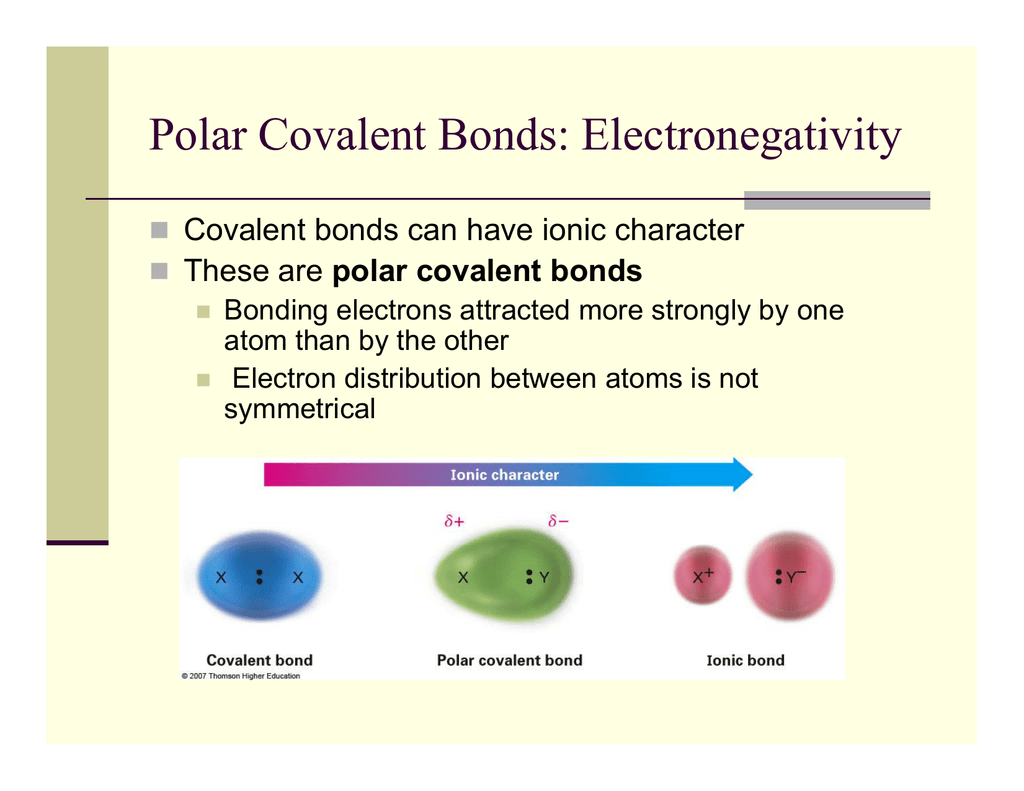
Polar Covalent Bonds Electronegativity

Is Hf Polar Or Nonpolar In Urdu Hindi Covalent Bond Electronegativity Youtube Youtube
Difference Between Covalent And Polar Covalent Definition Octet Rule Different Types
Pdf Chapter 4 Compounds And Their Bonds 4 6 Electronegativity And Bond Polarity
Comments
Post a Comment